| Issue |
SICOT-J
Volume 10, 2024
|
|
|---|---|---|
| Article Number | 35 | |
| Number of page(s) | 11 | |
| Section | Hip | |
| DOI | https://doi.org/10.1051/sicotj/2024031 | |
| Published online | 19 September 2024 | |
Congress Proceedings
How to start with hip arthroscopy in a safe and effective manner, using an evidence-based approach
1
Department of Orthopedic Surgery and Traumatology, Ghent University Hospital, Corneel Heymanslaan 10, 9000 Ghent, Belgium
2
Department of Trauma and Orthopedics, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Hills Road, Cambridge CB2 0QQ, UK
3
Mount Elizabeth Novena Hospital, 38 Irrawaddy Road, Singapore 329563, Singapore
4
Department of Orthopaedics, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Gothenburg 413 45, Sweden
5
Department of Orthopedics, Sri Lakshmi Narayana Institute of Medical Sciences, Puducherry 605502, India
6
Department of Orthopaedic Surgery and Traumatology, Faculty of Medicine, Assiut University, Assiut 71515, Egypt
7
Department of Orthopaedics and Traumatology, Kasr Alainy Hospital, Cairo Univerity, Cairo 11562, Egypt
8
The Institute of Orthopaedics “Carlos E. Ottolenghi”, Italian Hospital of Buenos Aires, Buenos Aires C1181ACH, Argentina
9
Orthopaedic Surgery and Traumatology Department, University Hospital Infanta Leonor, Madrid 28031, Spain
10
Department of Human Structure and Repair, Ghent University, Corneel Heymanslaan 10, 9000 Ghent, Belgium
* Corresponding author: emmanuel.audenaert@ugent.be
Received:
17
May
2024
Accepted:
2
August
2024
Hip arthroscopy is a rapidly evolving field in orthopedics, offering diagnostic and therapeutic benefits for a range of hip pathologies. This review outlines a comprehensive guide to initiating hip arthroscopy safely and effectively using evidence-based practices. Optimal surgical outcomes depend on correct indications for surgery, in particular in the presence of borderline dysplasia and degenerative joint diseases. Proper patient counseling and setting realistic expectations are crucial for satisfactory outcomes and recovery. Physical examination, radiographs, MRI, and CT scans are essential for accurate diagnosis. In case of diagnostic uncertainty, the use of intra-articular injections can help confirm the diagnosis before surgery. Techniques for hip arthroscopy include central compartment first, peripheral compartment first, and outside-in approaches. Each technique has advantages, and the optimal approach depends on the specific case. Finally, Proper operating room setup, meticulous patient positioning, and precise portal placement are critical for a successful procedure. A thorough understanding of the safe zone anatomy for portal placement is essential to minimize the risk of neurovascular complications. In conclusion, this manuscript provides a detailed, evidence-based framework for starting hip arthroscopy, emphasizing the importance of technical proficiency, patient selection, and a multidisciplinary approach to ensure patient safety and procedure efficacy.
Key words: Hip arthroscopy
© The Authors, published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Hip arthroscopy is a procedure still gaining in popularity, since 2009 the number of hip arthroscopies increased by 335–600% in the following five years [1, 2]. Because of the growing interest and developments, the management of femoro-acetabular impingement (FAI) is at a tipping point. We are shifting from a procedure performed by the pioneers and rapid adopters to a more broadly distributed usage of the technique and a more evidence-based approach. The rapid adoption of new procedures is now supported by stronger evidence, moving away from individual case reports or retrospective analyses to longer-term, prospective cohort trials [3].
Despite the growing interest in hip arthroscopy, the procedure is not one for the inexperienced surgeon. They need to master technical skills, the diagnostics, the pathophysiology, and the surgical indications. A multidisciplinary approach (physiotherapist, radiologist, orthopedic surgeon, and sports physician) is recommended for a safe arthroscopic procedure. As a surgeon, the technical skills can be optimized through a cadaver skills lab, simulation training, fellowship young adult hip, and a mentored independent practice. Hip arthroscopy is a procedure with a prolonged learning curve. Hoppe et al. saw a significant reduction in operation time and complication rates after 30 [4]. The learning curve could not be validated for 30 or any number of cases. The take-home message is: Master the technical skill, understand the pathology, and know your indication.
Pathophysiology
FAI is abnormal/premature contact of the proximal femur and the acetabulum, which can cause cartilage and labral damage and cause symptoms. These lesions can proceed in further degeneration and eventually in premature osteoarthrosis [5]. We can discriminate 3 forms of FAI: CAM impingement, pincer impingement, and combined impingement (Fig. 1) [6].
Cam impingement
Originally described as the pistol grip deformity by Stulberg et al. [7], is a decreased anterior femoral offset caused by a bony deformation of the anterolateral side of the femoral head/neck junction. The origin of the CAM can be idiopathic (femoro-acetabular retroversion, decreased pelvic incidence, genetic) or secondary (sports participation, slipped capital femoral epiphysiodesis, leg calve perthes, trauma, osteochondroma, infection, and osteoarthrosis) [6, 8, 9].
Pincer impingement
Over-coverage of the femoral head by the acetabulum can be caused by acetabular retroversion, which is more frequent in the female population. This is an over coverage of the femoral head anteriorly and an undercoverage posteriorly. Other causes are coxa profunda, protrusion acetabuli, and an overcorrecting peri-acetabular osteotomy (PAO). Because of the lever function, there is an impaction of the posteroinferior cartilage [6, 8, 9].
Mixed type
In 90% of the cases, there is a combined CAM-pincer combination.
Spinopelvic influence
The movement of the lumbar spine is in close connection with the movement of the hips especially in the sagittal plane. Patients with symptomatic FAI have less flexion in the spine and more in the hips. A lower pelvic incidence (PI) causes a decreased pelvic back tilt and accentuates contact between the acetabulum and the femur [10].
Evidence-based medicine approach to hip arthroscopy surgical indication
An optimal surgical result can be achieved by a good indication. Hip pain multiple DD (musculoskeletal, genitourinary, gynecological, neurological (L1L2L3) abdominal (appendicitis/hernia). Essentially everything starts with the physical examination. Upon inquiry groin dominant hip pain is important. The physical hip exam can be broken down into five parts; standing, seated, supine, lateral decubitus, and prone. Exams can include but are not limited to evaluating gait, alignment, strength, range of motion, palpation, version, and provocative testing. Upon physical examination, Flexion ADduction Internal Rotation (FADIR) is widely known. This test can be used as a screening tool when positive further examinations are needed. Radiographs are followed with MR to evaluate intra-articular damage. A CT can be valuable in evaluating bone deformations. The radial oblique sequence in the arthro mri is important in evaluating intra-articular pathology. For intra-articular problems, Arthro mri is superior to arthro CT, and arthro Ct superior to normal MRI 3T. Regarding the current investigations, a diagnostic arthroscopy is obsolete. When an intra-articular pathology is suspected an intra-articular injection is recommended. If the diagnostic infiltration gives no relief, the likelihood of relief in surgery decreases. This diagnostic algorithm is summarized in Figure 2 [6].
Satisfying postoperative outcomes is the result of the proper indication setting. This is best achieved by a team approach: the cooperation of the surgeon with the physiotherapist, radiologist, and sports physician. It is not only important to know the indications, but also the limitations of a hip arthroscopy (Table 1).
Indications for hip arthroscopy.
Hip biomechanics in femoro-acetabular impingement (FAI): how to improve with hip arthroscopy
Patient selection
When evaluating a patient with suspect FAI we will first look for a cam lesion on imaging to decide whether there is a clinically relevant cam. So, what is a clinically relevant cam lesion? Notably, impingement occurs with different portions of the cam during different activities of daily living (ADLs), and contact studies have demonstrated all cam lesions have the potential to be harmful [5].
In addition to cam morphology, assessment of overall hip geometry is equally crucial. Both acetabular and femoral morphotypes play a critical role in this regard. Any acetabular geometry that brings the acetabulum in closer proximity to the femoral neck increases the risk of cartilage damage, making deep socket and acetabular retroversion warning signs [11]. Similarly, any configuration that brings the femoral neck near the acetabulum raises the risk of harmful abutment. As such, both excessive femoral varus and femoral version have radiographic implications [12].
In summary, the decision to operate on cam morphology necessitates a comprehensive evaluation of cam, acetabular, and femoral geometry, all of which must be assessed in a 3D context.
Installation: the suction seal
To access the hip joint, a significant amount of traction force is necessary. To mitigate the risk of skin lesions and necrosis, a large, well-padded post will be utilized to distribute the traction forces. In addition, the Trendelenburg position can be employed to pull against gravity rather than pulling the patient against the post. The average force required for hip traction is approximately 714 Newtons, which is substantial [13].
The question of why the hip joint is so strongly sealed is seldom asked, but it may be a crucial factor in maintaining joint health. When walking, the weight of an average leg is roughly 15 kg, which translates to 150 N of force during simple ambulation. When this weight is combined with velocity, the distracting forces become even greater, with nearly 500 N of force during follow-through in soccer and up to 1000 N during a giant swing in gymnastics. Thus, the seal is a critical element in joint stability.
While a simplified vacuum model is frequently employed to explain the suction force involved in hip traction, the physics is more complicated than that. A crucial component herein involves wet adhesion. The mechanism of wet adhesion in synovial joints refers to the interaction of synovial fluid that forms a thin film with the cartilage surface [14]. This film acts as a reversible adhesive that can bond and debond with the cartilage surface depending on the applied load and shear stress. It is important for joint functioning because it prevents the joint surfaces from separating under mechanical stress. The mathematics behind this model is intricate, but several key points stand out in comparison to the simple vacuum model.
Firstly, the required force decreases as progressively more liquid bounds are broken [15]. Therefore, taking one’s time when applying traction can reduce the overall force required and the risk of skin tears. Secondly, the available joint surface is much more important than in a simplified vacuum model, with a quadratically higher impact on the force required for traction. Over-trimming should therefore be avoided, and torn labrum should be repaired as the available surface is critical to joint well-being and function. Finally, the properties of the synovial fluid, such as its viscosity and composition, can affect the force required to distract or separate the joint surfaces. Healthy synovial fluid will provide a stronger suction force, meaninga healthy jointdistracts much harder.
Hip joint stress following cam resection
Although there is significant evidence linking femoro-acetabular impingement (FAI), particularly cam lesions, to the development of hip osteoarthritis (OA), it remains difficult to demonstrate that a well-performed cam resection can prevent the occurrence of hip OA. Biomechanics can be utilized to assess the potential impact of hip arthroscopy, and research has shown that restoring femoral sphericity is crucial for normalizing joint pressures [16, 17]. However, there is a cautionary note for dysplasia. When the femoral head or acetabulum is not intrinsically spherical, reorienting the acetabulum can decrease cartilage loading but may not fully normalize it [18].
Importance of the hip capsule in joint kinetics
The ligaments and capsule are crucial components of the hip joint, and play a vital role in maintaining joint stability and endurance. The capsule not only restricts the joint from reaching extreme positions but also functions as a spring, assisting in returning the joint to its original position. The human body is highly energy-efficient, with approximately 70% of the energy cost for walking and running coming from elastic structures [19]. The strongest ligament in the body, the iliofemoral ligament, is a part of the anterior capsule. Research has shown that this ligament contributes significantly to walking and running, with approximately half the effort required during these activities. Therefore, when managing patients, especially athletes requiring physical endurance, it is important to minimize capsule damage and respect the role of the ligaments in maintaining physical endurance in the long term.
Operating room setup and portals to success in hip arthroscopy
Proper patient positioning and portal placement are important for an effective and safe hip arthroscopy. This is because of the proximity of neurovascular structures. There are two main patient positioning options: the supine position and the lateral decubitus position, each with its benefits and drawbacks. In the supine position, the non-operative limb is abducted to 45°, while the operative limb is, in order, abducted, traction given, internally rotated, and then adducted on the perineal post, to achieve both longitudinal and lateral distraction, as evidenced by the vacuum sign on the C-arm image. The advantages of the supine position include the familiar orientation of the joint for the surgeon and the ability to use a routine fracture table, while the disadvantages include difficult maneuverability in obese patients and decreased posterior access [20].
In contrast, the lateral decubitus position requires a specialized traction table. The advantages of this position include easy maneuverability in obese patients and better access to the posterior and inferior joint spaces. However, this position requires extra time and a special traction table.
In and around the hip there are 3 specific regions: 1) the central compartment, 2) the peripheral compartment (which are separated from each other by the margin of the acetabular labrum), and 3) the extra-articular compartment (peri trochanteric space) (Fig. 3) [21].
Based on which compartment, the arthroscope reaches first, the method of hip arthroscopy can be classified into three techniques: 1) a central compartment first technique, 2) a peripheral compartment first technique, and 3) an outside-in technique.
The central access first technique requires fluoroscopic guidance and adequate joint distraction, and provides direct access to the central compartment. However, it has certain drawbacks like longer traction time and hence higher risk of neuropraxia and the possibility of injury to the labrum or the cartilage.
The peripheral access first technique, popularized by Dienst et al., involves arthroscopic access to the anterior femoral neck region without traction, followed by entry into the central compartment under vision and traction [22, 23]. Flexion of the hip produces relaxation of the anterior capsule and allows easy scope entry into the central compartment. The advantages of the peripheral access first technique include a lower risk of injury to the labrum and cartilage, easier and safer access, less traction time, and usefulness when central access fails.
The outside-in technique involves access to the anterior extra-capsular space under fluoroscopy, followed by an anterior capsulotomy to access the peripheral compartment. The advantages of the outside-in technique include usefulness when intra-articular access is difficult due to adhesions, while drawbacks include being a technically demanding and extensive procedure relative to the other techniques, fluid extravasation, and risk of dislocation in shallow acetabulum and in the presence of ligament laxity.
Finally, it is important to note that there are several important portals to access the hip joint. Furthermore, it is important to note the safe zone: a rectangular area drown from ASIS to the posterior side of the greater trochanter and extending 5 cm more distal.
Some of the important portals are listed here (Fig. 4) [20, 23, 24]:
Anterolateral (AL) portal – 1 cm superior and 1 cm anterior to the tip of the greater trochanter
Anterior Portal (AP) – 1 cm lateral to the anterior superior iliac spine (ASIS) in line with the AL portal
Mid anterior/ Modified anterior portal (MAP)- Created by making an equilateral triangle between AL portal and anterior portal
DALA portal – Created by drawing an isosceles triangle between the MA and the AL portals and a point distal but in line with the AL portal
PALA portal – Junction of medial 1/3 and lateral 2/3 of line between the ASIS and the greater trochanter (GT)
Posterolateral portal (PL) – lies posterior of the top corner of the grater trochanter, passes through gluteus medius and minimus before arriving at the hip capsule.
Proximal Mid Anterior Portal (PMAP) – created making an equilateral triangle between Anterolateral and anterior portal to proximal (±1 cm distal of the vertical line through the ASIS).
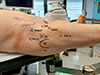 |
Figure 4 Portals of hip arthroscopy: Anterior Portal (AP), Anterolateral Portal (AL), Modified Anterior Portal (MAP), Proximal Mid Anterior Portal (PMAP), Proximal Accesory anterolateral portal (PALA), Distal Accessory Anterolateral portal (DALA) and posterolateral Portal (PL). |
Arthroscopic normal view and instruments to better achieve our goal in hip arthroscopy
A safe and effective hip arthroscopy starts with the adequate tools for your surgery. Following instruments are needed when starting with hip arthroscopy
Traction table
Well-padded post
Cannula for water flow
70° lens
Camera
Cannulated needle 14 gauge and nitinol wire
Cannulated switching stick
Half pipe
Cannulas 4.5 and 5.5 mm
Intra-articular knife
Shaver
Arthroscopic evaluation of the hip joint includes two sections that can be defined as the peripheral and central compartments [25].
The peripheral compartment can be assessed without traction and in different degrees of flexion to relax surrounding ligaments and musculature [26, 27]. A variety of portals have been used. Most often, proximal and distal anterolateral portals with the 70° lens. We prefer to start with the distal anterolateral portal for peripheral compartment assessment [28, 29]. Anteriorly, the femoral neck can be evaluated and is covered by a thin periosteal layer.
The 7-step routine evaluation of the hip joint is performed as described by Dienst, starting with the labrum; paralabral sulcus, medial synovial fold (MSF), Zona orbicularis, femoral neck and fat pad, femoral head, lateral synovial fold, and the psoas tendon. We propose the Said 9-step evaluation: which adds dynamic FAI evaluation and Labral probing [30]. Dynamic FAI evaluation of any Cam lesion is performed initially in 30 degrees of flexion with external/internal rotation then in further degrees of flexion as far as the traction system will allow usually 70–90 degrees. Anatomic landmarks are important to localize the pathology in the peripheral compartment. Referencing is done clockwise manner (Fig. 5). Taking the lateral hip at 12 o’clock and anterior at 3 o’clock in both hips. The subspinous bump is between 1 and 2 o’clock, the psoas tendon between 2 and 3 o’clock, and the MSF at 4 o’clock. Most of the cartilage lesions and labral lesions are located between 12 and 3 o’clock and the posterolateral impingement at 10 and 11 o’clock. Posteriorly, the reflected joint capsule with its vascular supply can be reached with flexion, adduction, and internal rotation [25]. These landmarks give excellent orientation within the peripheral compartment (Fig. 6).
 |
Figure 6 (A) Peripheral hip compartment assessment indicates femoral head (FH) and labrum (LA) as well as the Psoas tendon (PS). (B) Visualization of the subspinous bump/paralabral recess (SB), labrum (LA) and fhemoral head (FH). (C) Inspection of the femoral neck (FN), zona orbicularis (ZO) and medial synovial fold (MSF). |
Central compartment evaluation necessitates efficient traction to evaluate the space between the articular surface of the femoral head and that of the acetabulum. Central compartment is surrounded all around by the normal labrum. About 10 mm distraction is needed to allow free manipulation of 70ᵒ arthroscope and instruments [32]. For the central compartment, we add the direct anterolateral and occasionally the mid-anterior portals. With limited capsulotomies, this will provide full access to the central and peripheral compartments.
As in other joints, many mapping systems have been used to divide the articular cartilage into zones. For orientation and localization, the clock face nomenclature is used [25]. In the inferior part the central hip compartment, the horseshoe-shaped lunate surface is sealed by the transverse ligament that represents 6 o’clock on clock face mapping. The acetabular fossa is filled with fat and points to 12 o’clock, which refers to lateral articular cartilage. The ligamentum teres is an around cord-like structure that extends between the cotyloid fossa and the femoral head. Anteriorly at 3 o’clock, the psoas U-shape groove is a characteristic arthroscopic landmark [25]. The labrum, and chondrolabral junction is evaluated and probed as it surrounds the bony acetabular rim and it maintains congruity of the joint (Figs. 7 and 8).
 |
Figure 7 Central hip compartment assessment indicates the femoral head (FH), ligamentous teres (A), the posterior labrum (B) and the psoas U-shape groove at the 3 o’clock position. |
 |
Figure 8 Central hip compartment assessment indicates femoral head (FH), intact chondrolabral junction with wave sign/delamination (A), labral tear with disrupted chondrolabral junction (B), acetabular fossa pointing left towards 12 o’clock (C). |
Discussion
When hip arthroscopy is useful in borderline dysplasia
Developmental dysplasia of the hip includes a wide spectrum of conditions ranging from a high dislocation of the femoral head to a low-volume acetabulum in the young adult. Adult hip dysplasia, therefore, is not always a straightforward diagnosis, since subtle alterations in the acetabular volume/coverage will affect the biomechanics of the hip joint resulting in hip instability and progression to intra-articular degeneration, while not producing significant initial radiological changes. Historically, a lateral center-edge angle (LCEA) below 20°, measured on an anteroposterior pelvic radiograph, has been used to categorize a dysplastic hip as such [33].
While hip dysplasia commonly corresponds with a deficient anterior-lateral wall coverage of the femoral head, one in six cases are associated with posterior wall deficiency (i.e., acetabular retroversion) [34]. It has been shown that such acetabular wall deficiencies, either anterior or posterior, may exist even with normal or so-called borderline lateral wall coverage [35]. There are also signs of missed micro-instability, i.e., upsloping of the lateral sourcil [36].
While different classification systems have been widely adopted to categorize the degree of hip dysplasia in the setting of end-stage osteoarthritis (e.g., Crowe’s or Hartofilakidis’ classifications), there are still no popularized classification systems for the pre-arthritic dysplastic hip in the young adult. Based on the LCEA, some have classified dysplasia as evident when LCEA is below 20°, and “borderline” when between 20 and 25°, considering “normal” values as those lying between 25 and 40°. While many surgeons have treated (and still treat) “borderline” dysplasia cases with hip arthroscopy alone, there is current evidence showing that such cases usually present with other features of dysplasia, and thus the terms “mild” or “borderline” may be inappropriate, with a containment procedure (i.e., periacetabular osteotomy [PAO]) better addressing the source of pain [37].
Considering that isolated analysis of the LCEA may lead to under-diagnosis of hip dysplasia, Wilkin et al., using acetabular wall indices’ measurement [38] together with LCEA and Tönnis angle measurement, have proposed a novel classification system: global/lateral dysplasia, pure anterior wall-deficient dysplasia, and pure posterior wall-deficient dysplasia [39]. The authors of the same article have reported good-to-excellent inter and intra-observer reliability using this classification [40]. With this classification, the terms “borderline” or “mild” are eliminated for their ambiguity, with hips being considered dysplastic or non-dysplastic based on 360° femoral head wall coverage analysis. Nonetheless, this classification system is based on plain radiographs only. Therefore, to better delineate the exact location of a wall deficiency, Nahal et al. have described 3D-CT acetabular wall coverage analysis with acetabular sector angle (ASA) measurement as an effective tool to characterize dysplastic acetabular morphology [41]. With this approach, anterior and posterior ASAs help the hip preservation surgeon understand the pattern of deficiency, which is essential for preoperative planning of a re-orientational procedure.
One of the case-presenters (PAS) of the past SICOT Webinar (March 30th, “360° Hip View: from Preserving to Revision THA – How to Start with Hip Arthroscopy in a Safe and Effective Manner”) presented a case of a failed borderline dysplasia initially treated with hip arthroscopy which was salvaged with a combined new hip arthroscopy + PAO. Although more evidence is still needed on the effectiveness of concomitant hip arthroscopy as an adjuvant procedure for PAO, the author of this report believes that it might be valuable in cases with previous surgery (to discard intra-articular adhesions or iatrogenic injuries), in patients, 45 years of age (to discard advanced hip arthritis) and in those with acetabular retroversion, as previously described [42]. As others have shown, PAO is, up to date, the best treatment option for so-called borderline dysplasia cases [43], and hip arthroscopy should not be used as a sole treatment for such cases, since it might increase instability and accelerate the development of secondary arthritis.
How much degenerative changes are too much
Preservation surgery, especially arthroscopy, role in degenerative joint diseases (DJD) in all joints is a matter of high debate. Under the title “How much degenerative change is too much?”, we discussed the management of a case presentation of a 28-year-old female with normal body mass index suffering from combined femoro-acetabular impingement (FAI) with degenerative joint disease (DJD) (Fig. 9).
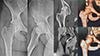 |
Figure 9 The full imaging profile needed for proper decision-making of a 28-year-old female presenting with right hip pain for two years. Plain weight-bearing radiographs (right), CT (upper left) and MRA (bottom left) reveal combined FAI with DJD. |
Preservation surgery, especially arthroscopy, in DJD in all joints depends on several factors. No doubt, its role in the late stages is negligible. However, the literature is always debatable about the early stages [44]. In the hip joint, the literature reports several factors that should be considered in decision-making and outcome expectations. Most of these factors can be gathered preoperatively from a combination of the clinical examination, especially the painful range of motion, with plain weight-bearing radiographs, computed tomography and high-resolution magnetic resonance imaging or arthrography (MRI or MRA) [45, 46].
Five factors found consensus. Patient’s presenting in the fifth decade of life and older are considered at high risk of poor prognosis, even if the arthritic changes are early. Acetabular hyaline cartilage condition is a very important factor; it depends on the degree of damage and site. In general, deep lesions with MRI Outerbridge ≥ 2 are associated with unfavourable results. Moreover, if it is located in the weight-bearing surface area, this is an additive strong poor prognostic factor contrary to the peripherally located lesion. The radiographic degree of joint arthritic changes, especially if the joint space narrowing < 2 mm or its grade is more than two, according to Tönnis, is of poor outcome. Finally, the degree of damage to the femoral head must be considered. Poor prognosis is excepted when the femoral heads show chondral lesions, subchondral cysts, and oedema [46].
Another matter of debate is surgical management and what to do with these hips. As preservation surgeons, we are always keen on repairing and reconstructing the damaged labrum or hyaline cartilage; however, excision and debridement are performed here more, due to their degenerative nature and fear of the persistence of pain after the operation (Fig. 10). This is combined with the classical excision of acetabular over coverage and femoral bumpectomy, if needed, to stop further mechanical damage [44–46].
 |
Figure 10 Intraoperative photos show the degenerative changes in the labrum and the peripheral hyaline cartilage (A). A trail of loop suture ripped and cut through the degenerated labrum (B). Finally, partial labrectomy and acetabulum recession were done (C). |
Of utmost importance, the webinar panel agreed that these patients’ results are always below the level of their expectations and hopes, especially in young active ones. We recommend proper patient counselling and avoidance of false, unrealistic expectations [46, 47].
Conclusion
Hip arthroscopy has proven to be a safe and efficient procedure for diagnosing and treating various hip pathologies, despite a significant learning curve. The procedure demands a comprehensive understanding of technical skills, diagnostics, and pathophysiology. For the starting arthroscopist, adhering to evidence-based practices and involving a multidisciplinary team enhances safety and efficacy. Proper patient selection, meticulous preoperative planning, and precise intraoperative techniques are critical. The importance of appropriate patient positioning, portal placement, and traction management cannot be overstated. By considering these aspects, hip arthroscopy can be performed safely, even by those new to the procedure.
Funding
Emmanuel Audenaert was financially supported by a senior clinical research fellowship from the Research Foundation Flanders.
Conflicts of interest
The authors declare that they have no relevant financial or non-financial interests to report.
Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request. Data supporting the findings of this study may be subject to certain restrictions due to privacy or ethical considerations. Any requests for data sharing will be reviewed on a case-by-case basis in accordance with institutional guidelines.
Author contribution statement
All authors contributed equally to the writing, editing and reviewing of this manuscript.
Ethics approval
Ethical approval was not required.
References
- Bozic KJ, Chan V, Valone FH 3rd, Feeley BT, Vail TP (2013) Trends in hip arthroscopy utilization in the United States. J Arthroplasty 28(8 Suppl), 140–143. [CrossRef] [PubMed] [Google Scholar]
- Montgomery SR, Ngo SS, Hobson T, Nguyen S, Alluri R, Wang JC, Hame SL (2013) Trends and demographics in hip arthroscopy in the United States. Arthroscopy 29(4), 661–665. [CrossRef] [PubMed] [Google Scholar]
- Khan M, Ayeni OR, Madden K, Bedi A, Ranawat A, Kelly BT, Sancheti P, Ejnisman L, Tsiridis E, Bhandari M (2016) Femoroacetabular impingement: have we hit a global tipping point in diagnosis and treatment? Results from the international femoroacetabular impingement optimal care update survey (IN FOCUS). Arthroscopy 32(5), 779–787. [CrossRef] [PubMed] [Google Scholar]
- Hoppe DJ, de Sa D, Simunovic N, Bhandari M, Safran MR, Larson CM, Ayeni OR (2014) The learning curve for hip arthroscopy: a systematic review. Arthroscopy 30(3), 389–397. [CrossRef] [PubMed] [Google Scholar]
- Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA (2003) Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 417, 112–120. [Google Scholar]
- Khan M, Bedi A, Fu F, Karlsson J, Ayeni OR, Bhandari M (2016) New perspectives on femoroacetabular impingement syndrome. Nat Rev Rheumatol 12(5), 303–310. [CrossRef] [Google Scholar]
- Stulberg SD (1975) Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: The hip: proceedings of the third open scientific meeting of the hip society. Cordell LD, Harris WH, Ramsey PL, MacEwen GD, Editors. St Louis, MO: CV Mosby. p. 212–228. [Google Scholar]
- Byrd JW, Jones KS (2011) Arthroscopic management of femoroacetabular impingement in athletes. Am J Sports Med 39(1_Suppl), 7S–13S. [CrossRef] [Google Scholar]
- Morris W, Li R, Liu R, Salata M, Voos J (2017) Origin of cam morphology in femoroacetabular impingement. Am J Sports Med 46(2), 478–486. [Google Scholar]
- Grantham W, Philippon M (2019) Etiology and pathomechanics of femoroacetabular impingement. Curr Rev Musculoskel Med 12(3), 253–259. [CrossRef] [Google Scholar]
- Houcke JV, Khanduja V, Pattyn C, Audenaert E (2017) The history of biomechanics in total hip arthroplasty. Indian J Orthop 51(4), 359–367. Erratum in: Indian J Orthop 2017;51(5):629. [CrossRef] [PubMed] [Google Scholar]
- Van Houcke J, Audenaert EA, Atkins PR, Anderson AE (2020) A combined geometric morphometric and discrete element modeling approach for hip cartilage contact mechanics. Front Bioeng Biotechnol 21(8), 318. [CrossRef] [PubMed] [Google Scholar]
- Röling MA, Mathijssen NM, Blom I, Lagrand T, Minderman D, Bloem RM (2019) Traction force for peroperative hip dislocation in hip arthroscopy. HIP Int 30(3), 333–338. [Google Scholar]
- van der Spoel D, Wensink EJ, Hoffmann AC (2006) Lifting a wet glass from a table: a microscopic picture. Langmuir 22(13), 5666–5672. [CrossRef] [PubMed] [Google Scholar]
- Chen Y, Meng J, Gu Z, Wan X, Jiang L, Wang S (2019) Bioinspired multiscale wet adhesive surfaces: structures and controlled adhesion. Adv Funct Mater 30(5), 1905287. [Google Scholar]
- Van Houcke J, Khanduja V, Audenaert EA (2021) Accurate arthroscopic cam resection normalizes contact stresses in patients with femoroacetabular impingement. Am J Sports Med 49(1), 42–48. [CrossRef] [PubMed] [Google Scholar]
- Van Houcke J, Khanduja V, Nakano N, Krekel P, Pattyn C, Audenaert E (2017) Accuracy of navigated cam resection in femoroacetabular impingement: a randomised controlled trial. Int J Med Robot 13(4), e1839. [CrossRef] [Google Scholar]
- Goetz JE, Thomas-Aitken HD, Sitton SE, Westermann RW, Willey MC (2023) Joint contact stress improves in dysplastic hips after periacetabular osteotomy but remains higher than in normal hips. Hip Int 33(2), 298–305. [CrossRef] [PubMed] [Google Scholar]
- Duquesne K, Pattyn C, Vanderstraeten B, Audenaert EA (2022) Handle With Care: the anterior hip capsule plays a key role in daily hip performance. Orthop J Sports Med., 10(3), 23259671221078254. [CrossRef] [Google Scholar]
- Marin-Peña O, Lund B, Ayeni OR, Dantas P, Griffin D, Khanduja V, Said HG, Tey M, Dickenson E, Kay J, Mascarenhas V, Sadakah MA, Sunil KH, Tahoun M (2018) Basic concepts in hip arthroscopy. In: ESSKA instructional course lecture book. Kerkhoffs GMMJ, Haddad F, Hirschmann MT, Karlsson J, Seil R, Editors. Springer: Berlin, Heidelberg. p. 45–67. [CrossRef] [Google Scholar]
- Themes UFO (2016) Peripheral compartment approach to hip arthroscopy, musculoskeletal key. Available at: https://musculoskeletalkey.com/peripheral-compartment-approach-to-hip-arthroscopy/ (accessed: April 20, 2023). [Google Scholar]
- Dienst M, Seil R, Kohn DM (2005) Safe arthroscopic access to the central compartment of the hip. Arthroscopy 21(12), 1510–1514. [CrossRef] [PubMed] [Google Scholar]
- Tang H-C, Brockwell J, Dienst M (2020) Hip arthroscopy via a peripheral compartment first capsular-preserving technique: a step-by-step description. J Hip Preserv Surg 7(3), 596–603. [Google Scholar]
- Perry AK, DeFroda SF, Gursoy S, Murray IR, Vadhera AS, Nho SJ, Chahla J (2021) Top ten pearls for successful hip arthroscopy for femoroacetabular impingement. Arthrosc Tech 10(8), e2033–e2042. [CrossRef] [PubMed] [Google Scholar]
- Griffin D, Karthikeyan S (2012) Normal and pathological arthroscopic view in hip arthroscopy. In: Femoroacetabular impingement. Marín-Peña Ó, Editor. Springer-Verlag: Berlin, Heidelberg. p. 113–122. [CrossRef] [Google Scholar]
- Beck M (2009) Groin pain after open FAI surgery: the role of intraarticular adhesions. Clin Orthop Relat Res 467, 769–774. [CrossRef] [PubMed] [Google Scholar]
- El-Radi MA, Marin-Peña OR, Said HG, Tey-Pons M (2017) Basics in hip chondrolabral lesions and state of the art. SICOT J 3, 73. [CrossRef] [EDP Sciences] [Google Scholar]
- Barberá OF, Navarro IS (2013) Gross anatomy. In: Operative hip arthroscopy. Thomas Byrd JW, eds. Berlin, Heidelberg: Springer-Verlag. [Google Scholar]
- Marin-Peña O, Lund B, Ayeni OR, Dantas P, Griffin D, Khanduja V, Said HG, Tey M, Dickenson E, Kay J, Mascarenhas V, Sadakah MA, Sunil Kumar KH, Tahoun M (2018) Basic concepts in hip arthroscopy. Glasgow: ESSKA Instructional Course Lecture Book. [Google Scholar]
- Apivatgaroon A, Dienst M (2016) Compression and flip test for diagnosis of unstable acetabular labral tears using a peripheral compartment approach. Arthroscopy Tech 5(6), e1433–e1439. [CrossRef] [Google Scholar]
- Tian C-Y, Wang J-Q, Zheng Z-Z, Ren A-H (2014) 3.0T conventional hip mr and hip mr arthrography for the acetabular labral tears confirmed by arthroscopy. Eur J Radiol 83(10), 1822–1827. [CrossRef] [PubMed] [Google Scholar]
- Shetty VD, Villar RN (2007) Hip arthroscopy: current concepts and review of the literature. Br J Sports Med 41(2), 64–68. [CrossRef] [PubMed] [Google Scholar]
- Clohisy JC, Carlisle JC, Beaulé PE, et al. (2008) A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am 90(Suppl 4), 47–66. [CrossRef] [PubMed] [Google Scholar]
- Slullitel PA, Grammatopoulos G, Smit K, et al. (2021) Hip joint preservation surgery: what every orthopaedic provider needs to know. Instr Course Lect 70, 181–208. [PubMed] [Google Scholar]
- McClincy MP, Wylie JD, Yen Y-M, Novais EN (2019) Mild or borderline hip dysplasia: are we characterizing hips with a lateral center-edge angle between 18° and 25° appropriately? Am J Sports Med 47, 112–122. [CrossRef] [PubMed] [Google Scholar]
- Wong TY, Jesse MK, Jensen A, Kraeutler MJ, Coleman C, Mei-Dan O (2018) Upsloping lateral sourcil: a radiographic finding of hip instability. J Hip Preserv Surg 5(4), 435–442. [CrossRef] [PubMed] [Google Scholar]
- McClincy MP, Wylie JD, Kim Y-J, et al. (2019) Periacetabular osteotomy improves pain and function in patients with lateral center-edge angle between 18° and 25°, but are these hips really borderline dysplastic? Clin Orthop Relat Res 477, 1145–1153. [CrossRef] [PubMed] [Google Scholar]
- Siebenrock KA, Kistler L, Schwab JM, et al. (2012) The acetabular wall index for assessing anteroposterior femoral head coverage in symptomatic patients. Clin Orthop Relat Res 470, 3355–3360. [CrossRef] [PubMed] [Google Scholar]
- Wilkin GP, Ibrahim MM, Smit KM, Beaulé PE (2017) A contemporary definition of hip dysplasia and structural instability: toward a comprehensive classification for acetabular dysplasia. J Arthroplasty 32, S20–S27. [CrossRef] [PubMed] [Google Scholar]
- Bali K, Smit K, Ibrahim M, et al. (2020) Ottawa classification for symptomatic acetabular dysplasia assessment of interobserver and intraobserver reliability. Bone Joint Res 9, 242–249. [CrossRef] [PubMed] [Google Scholar]
- Nahal C, Slullitel PA, Kamenaga T, et al. (2022) Acetabular coverage analysis of the proximal femoral head accurately characterizes dysplastic acetabular morphology. J Orthop Res 41(6), 1273–1282. [Google Scholar]
- Sabbag CM, Nepple JJ, Pascual-Garrido C, et al. (2019) The addition of hip arthroscopy to periacetabular osteotomy does not increase complication rates: a prospective case series. Am J Sports Med 47, 543–551. [CrossRef] [PubMed] [Google Scholar]
- Nepple JJ, Parilla FW, Pashos GE, Clohisy JC (2023) Outcomes of periacetabular osteotomy for borderline acetabular dysplasia. J Bone Joint Surg Am 105, 137–144. [CrossRef] [PubMed] [Google Scholar]
- Cross GWV, Sobti AS, Khan T (2021) Hip arthroscopy in osteoarthritis: is it an option? J Clin Orthop Trauma 30(22), 101617. [CrossRef] [PubMed] [Google Scholar]
- Kemp JL, MacDonald D, Collins NJ, Hatton AL, Crossley KM (2015) Hip arthroscopy in the setting of hip osteoarthritis: systematic review of outcomes and progression to hip arthroplasty. Clin Orthop Relat Res 473(3), 1055–1073. [CrossRef] [PubMed] [Google Scholar]
- Mella C, Villalón IE, Núñez Á, Paccot D, Díaz-Ledezma C (2015) Hip arthroscopy and osteoarthritis: where are the limits and indications? SICOT J 16(1), 27. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Chaudhry ZS, Salem HS, Hammoud S, Salvo JP (2019) Does prior hip arthroscopy affect outcomes of subsequent hip arthroplasty? A systematic review Arthroscopy 35(2), 631–643. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Follet L, Khanduja V, Thevendran G, Ayeni O, Shanmugasundaram S, Abd El-Radi M, Said H, Abdelazeem A, Slullitel P, Marin-Peña O & Audenaert E (2024) How to start with hip arthroscopy in a safe and effective manner, using an evidence-based approach. SICOT Webinar 2023. SICOT-J 10, 35
All Tables
All Figures
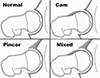 |
Figure 1 Anatomic variances leading tot femoro-acetabular impingement [6]. |
| In the text | |
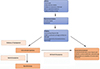 |
Figure 2 Algorithmic approach for a safe arthroscopic indication [6]. |
| In the text | |
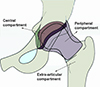 |
Figure 3 Different compartments of hip arthroscopy [21]. |
| In the text | |
 |
Figure 4 Portals of hip arthroscopy: Anterior Portal (AP), Anterolateral Portal (AL), Modified Anterior Portal (MAP), Proximal Mid Anterior Portal (PMAP), Proximal Accesory anterolateral portal (PALA), Distal Accessory Anterolateral portal (DALA) and posterolateral Portal (PL). |
| In the text | |
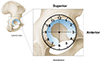 |
Figure 5 Schematic drawing of the clockwise referencing of the acetabulum [31]. |
| In the text | |
 |
Figure 6 (A) Peripheral hip compartment assessment indicates femoral head (FH) and labrum (LA) as well as the Psoas tendon (PS). (B) Visualization of the subspinous bump/paralabral recess (SB), labrum (LA) and fhemoral head (FH). (C) Inspection of the femoral neck (FN), zona orbicularis (ZO) and medial synovial fold (MSF). |
| In the text | |
 |
Figure 7 Central hip compartment assessment indicates the femoral head (FH), ligamentous teres (A), the posterior labrum (B) and the psoas U-shape groove at the 3 o’clock position. |
| In the text | |
 |
Figure 8 Central hip compartment assessment indicates femoral head (FH), intact chondrolabral junction with wave sign/delamination (A), labral tear with disrupted chondrolabral junction (B), acetabular fossa pointing left towards 12 o’clock (C). |
| In the text | |
 |
Figure 9 The full imaging profile needed for proper decision-making of a 28-year-old female presenting with right hip pain for two years. Plain weight-bearing radiographs (right), CT (upper left) and MRA (bottom left) reveal combined FAI with DJD. |
| In the text | |
 |
Figure 10 Intraoperative photos show the degenerative changes in the labrum and the peripheral hyaline cartilage (A). A trail of loop suture ripped and cut through the degenerated labrum (B). Finally, partial labrectomy and acetabulum recession were done (C). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


